- Posted On:2023-07-13 14:07
-
888 Views
FDA approves the first over-the-counter birth control pill
For the first time, the Food and Drug Administration has approved a birth control pill to be sold without a prescription, meaning it will be available to millions of people over the counter at pharmacies, convenience stores, and grocery stores around the country, as well as online.
The FDA's approval, announced Thursday morning, is seen as a victory for the sexual and reproductive rights of Americans, which are currently under intense attack in much of the country.
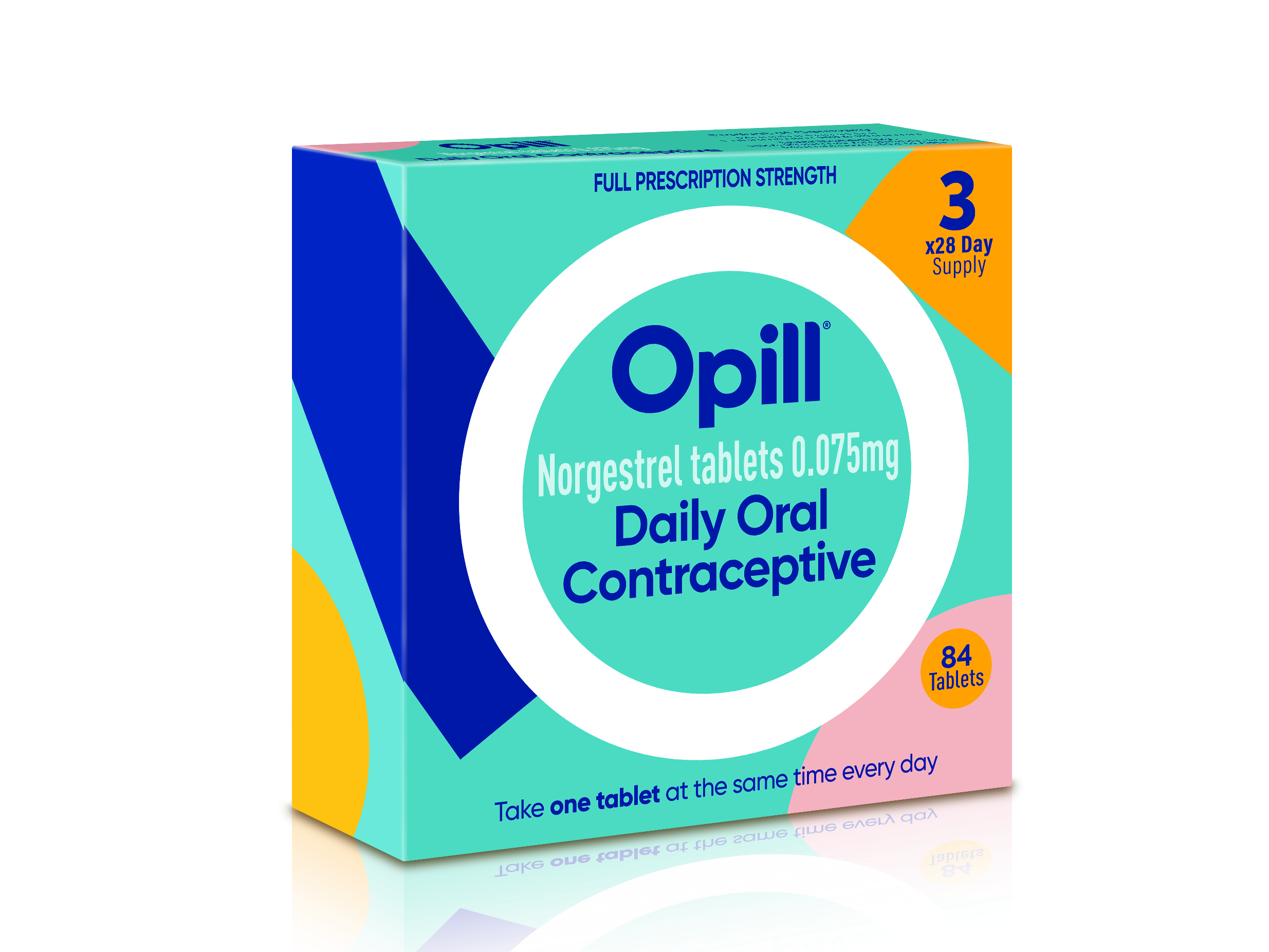
The OTC-approved pill is the Opill (norgestrel), a once-a-day progestrin-only pill manufactured by the Dublin-based company Perrigo. The company said it expects the pill will be available starting in the first quarter of 2024, though its pricing is not yet clear. For optimal efficacy, it needs to be taken consistently every day in the same three-hour window. Opill is estimated to be about 93 percent effective at preventing pregnancy in real-life use, higher than the real-life efficacy of other over-the-counter birth control methods, such as condoms, which are around 87 percent effective.
"Today’s approval marks the first time a nonprescription daily oral contraceptive will be an available option for millions of people in the United States," Patrizia Cavazzoni, director of the FDA’s Center for Drug Evaluation and Research, said in a statement. "When used as directed, daily oral contraception is safe and is expected to be more effective than currently available nonprescription contraceptive methods in preventing unintended pregnancy.”
Major medical and health organizations—including the American Medical Association, the American College of Obstetricians and Gynecologists, and the American Academy of Family Physicians—have previously endorsed OTC availability of oral birth control. And the FDA's approval today follows a May advisory committee meeting in which independent experts voted unanimously (17 to 0) in support of the approval.
“A momentous day”
The vote came after the FDA advisers spent two days going over all of the safety and efficacy data related to the pill. "The risks to women of an unintended pregnancy are much greater than any of the things we were discussing as risks of putting this pill out over the counter," panel member Katalin Roth, a medical professor at George Washington University, said in closing remarks at the May meeting. "The history of women's contraception is a struggle for women's control over their reproduction, and we need to trust women."
In a statement, Patrick Lockwood-Taylor, the president and CEO of Opill's maker, Perrigo, said, "Today marks a truly momentous day for women's health nationwide," adding that it "has the potential to radically transform women's access to contraception."
The price point for the OTC birth control pill will be the next critical factor for its easy access. Perrigo has not publicly announced a price, but Perrigo Global Vice President for Women's Health Frederique Welgryn said in a statement that the company is "committed" to making Opill "accessible and affordable to women and people of all ages."
Meanwhile, the Biden administration and Democratic lawmakers have pushed for and proposed legislation to require insurers to cover over-the-counter contraception.
Almost half of the country's 6.1 million pregnancies each year are unintended pregnancies, the FDA noted. And unintended pregnancies are linked to harms to both the pregnant person and infants, including reduced likelihood of receiving early prenatal care and increased risk of preterm delivery, which is linked to complications in newborns, as well as adverse developmental and child health outcomes later.
With the removal of the constitutional right to abortion access last year and the resulting deluge of bans and restrictions in many states, the risks of unintended pregnancies have only grown. As such, reproductive rights advocates cheered today's approval.
"Birth control is essential health care," Alexis McGill Johnson, president and CEO of Planned Parenthood Federation of America, said in a statement. "Today’s FDA decision is a historic moment for health equity, sexual health, and reproductive rights. … We are thrilled to see the FDA follow the science and remove an unnecessary barrier to accessing basic health care."