- Posted On:2023-10-03 17:10
-
870 Views
Probiotic bacterium kills preterm infant; FDA blasts supplement maker
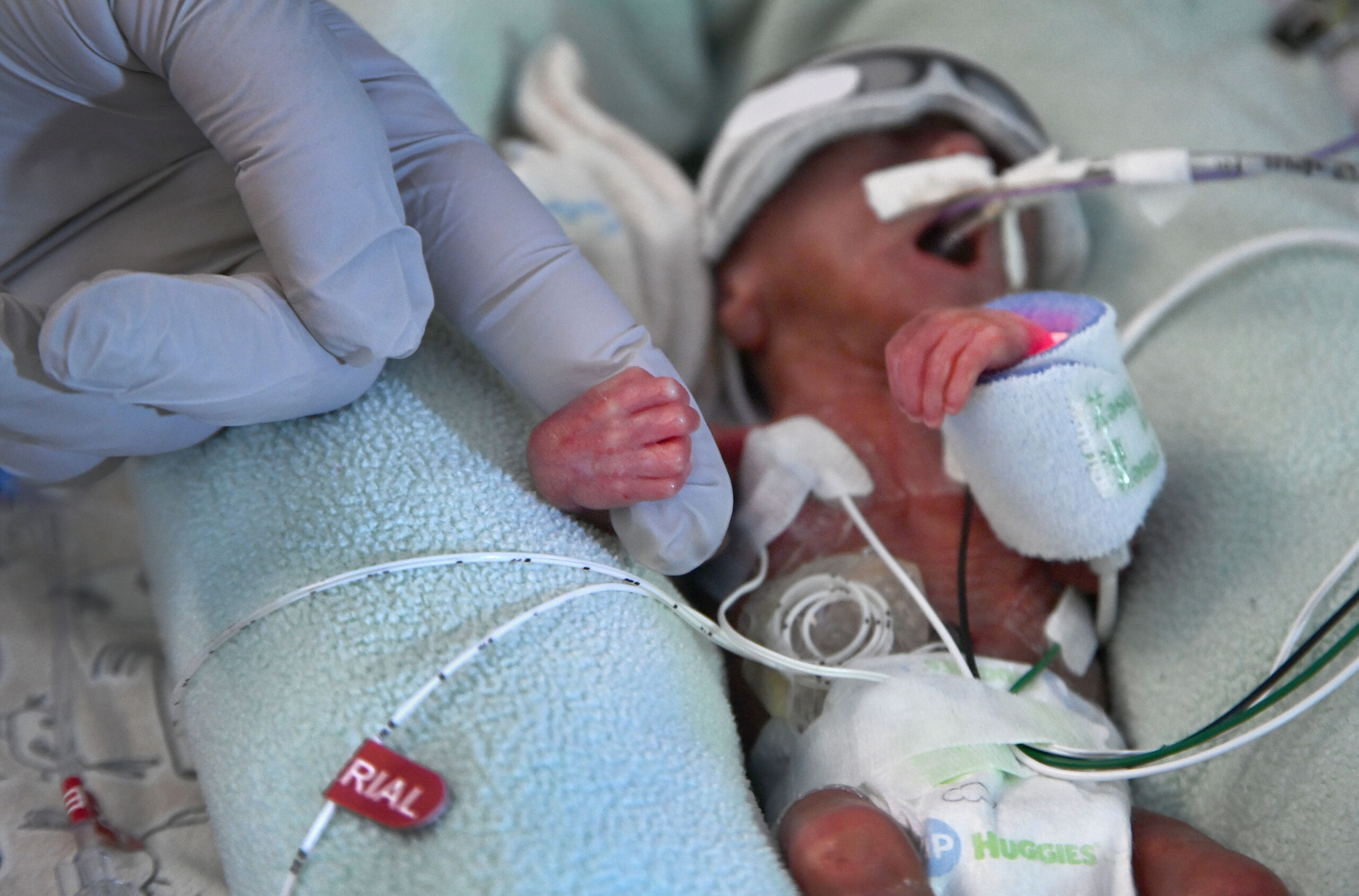
The Food and Drug Administration is warning health care providers not to use probiotics containing live bacteria or yeast in preterm infants after the agency began investigating the July death of a preterm, low-weight infant given such a product in an unnamed hospital.
The infant developed sepsis from the bacterium in the probiotic product—Evivo with MCT Oil made by Infinant Health—and subsequently died.
In a statement to Ars, the FDA said it quickly investigated the death after receiving an initial report on July 31. "Infant deaths are especially tragic and determining causality of preterm infant death can be particularly complicated," an agency spokesperson said. The agency reviewed medical records and laboratory tests from the case and collected clinical samples and product samples for analysis.
"In September 2023, genomic sequencing data analysis performed by the FDA found that the probiotic bacteria present in Evivo with MCT Oil was a genetic match to the bacterium isolated from the infant’s blood," the agency said.
The bacteria in the product and found in the infant was Bifidobacterium longum subspecies infantis.
The FDA did not respond to Ars' question about the state or hospital where the death occurred.
On Friday, the FDA sent out its warning to health care providers about the dangers of probiotics in infants, particularly preterm infants that are highly vulnerable. The agency noted that the case isn't the first of its kind; there have been previous reports of infections and sepsis in infants due to the use of probiotics containing bacteria and yeast.
"The FDA is also reminding healthcare providers that FDA has not approved any probiotic product for use as a drug or biological product in infants," the agency wrote in the warning.
Unproven, risky treatment
Probiotics are generally not recommended for infants, particularly vulnerable ones. The American Academy of Pediatrics recommends against them. "Given the lack of FDA-regulated pharmaceutical-grade products in the United States, conflicting data on safety and efficacy, and potential for harm in a highly vulnerable population, current evidence does not support the routine, universal administration of probiotics to preterm infants, particularly those with a birth weight of <1000 g." Still, a 2021 report found that approximately 10 percent of very premature infants in the US receive some sort of probiotic preparation while receiving specialized care in hospitals.
While the FDA tried to inform health care providers about the dangers of probiotics, the agency also sent a rebuking letter to the maker of Evivo with MCT Oil: Infinant Health, Inc., formerly Evolve Biosystems Inc. The agency criticized the California-based company for clearly marketing its unapproved product—sold as a dietary supplement—for use in the prevention of a serious disease in highly vulnerable pre-term infants. Moreover, the agency took aim at the company touting the supplement as "made specifically for use in healthcare settings," and "[d]esigned specifically for infants in the NICU[.]" NICU is the Neonatal Intensive Care Unit, where the most vulnerable of newborns, many preterm, receive specialized and intensive care.
Preterm infants are those born after less than 37 weeks of gestation and are at high risk for morbidity and mortality, the FDA notes. "Because their gastrointestinal system is not fully matured, preterm infants have more permeable intestinal linings, often referred to as 'leaky guts,' and motility problems, which can lead to opportunistic infections and sepsis when ingesting living microorganisms."
Infinant Health claims that its product can prevent necrotizing enterocolitis, a life-threatening inflammation of the large intestine that plagues newborns, particularly those who are preterm and low weight. However, published data do not support the claim, and Infinant Health's product has not gone through the FDA's pre-market review of safety and efficacy. The FDA has also not granted approval for its use in clinical trials.
In all, the FDA's warning letter to Infinant Health stated that, based on federal regulations, Evivo with MCT Oil constituted both an unapproved new drug and an adulterated food. But it's unclear if Infinant was previously aware of this.
Multiple meetings
The FDA's warning letter to Infinant Health notes that the company met with the FDA in early July, before the infant's death. In the meeting, the FDA expressed concerns about the company's products.
"FDA communicated these safety concerns related to use in food for preterm infants during a meeting between the FDA’s Center for Food Safety and Applied Nutrition (CFSAN), Office of Food Additive Safety’s Division of Food Ingredients and Infinant Health on July 7, 2023," the FDA wrote. "In particular, FDA questioned whether the clinical studies Infinant cited in its presentation to FDA were robust enough to support safety, as the studies were short-term, poorly designed, and difficult to interpret without appropriate controls."
The FDA declined multiple times to respond to Ars' questions about this meeting, including why it occurred and if it resulted in any FDA actions.
The FDA's letter to Infinant notes that the agency and the company met again in September. The FDA seemed to suggest that, rather than heeding the regulator's concerns about its product, Infinant was digging in its heels and possibly making more unsupported claims that the FDA may not know about.
"During a meeting with FDA on September 21, 2023, your firm emphasized to Agency representatives the benefits of B. longum subsp. infantis in Evivo with MCT Oil for preterm infants in neonatal intensive care units, including by reducing the incidence of necrotizing enterocolitis. These statements suggest that similar claims may exist in other labeling or promotional materials that may be inaccessible to FDA."
Infinant Health did not immediately respond to questions from Ars.
A spokesperson for the company responded to CBS News, however, saying that Infinant Health is planning to continue distributing its "Evivo powder product" for consumers to buy and intends "to work with the FDA toward approval of the use of our MCT oil product in hospital settings."